Introduction: Next-generation sequencing (NGS) represents a promising modality for measurable residual disease (MRD) testing in patients with acute myeloid leukemia (AML), but systematic efforts are required to define the appropriate targets, test characteristics, and clinical implications. We recently reported that detection of persistent NPM1 or FLT3 internal tandem duplication (ITD) mutations in adults with AML in first complete remission (CR1) prior to allogeneic hematopoietic cell transplantation (alloHCT) is associated with increased relapse and death compared with those testing negative (JAMA 2023, PMID: 36881031). FLT3 tyrosine kinase domain (TKD) variants are present in 7-10% of adult patients with AML but the utility of this target for AML MRD testing in CR1 prior to alloHCT was previously unknown.
Methods: Patients aged 18 or older who underwent alloHCT for AML in CR1 between 2013 and 2019 reported to have a FLT3-TKD variant at diagnosis and a pre-conditioning remission blood sample available in the CIBMTR biorepository were eligible for this study. Single-amplicon NGS (SA-NGS) library generation utilizing UMI-tagged primers targeting the D835 and I836 codons of FLT3 was performed on 400ng genomic DNA sequenced with unique dual indices, with error-corrected variant calling. Primary outcomes were overall survival (OS) and cumulative incidence of relapse (CIR). Secondary outcomes were relapse-free survival (RFS) and non-relapse mortality (NRM), with NRM being considered as a competing risk.
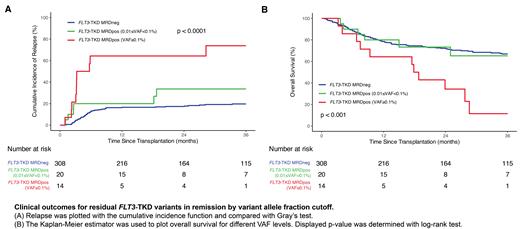
Results: A total of351 patients met inclusion criteria for this study, and 342 (97.4%) had sufficient DNA for SA-NGS and were analyzed for clinical outcomes. Of these patients, 81 (23.7%) experienced relapse at a median of 5 months post-alloHCT. Using the current ELN-recommended variant allele fraction (VAF) threshold of 0.1% for NGS-based AML MRD, 14 patients (4.1%) tested positive. Patients with FLT3-TKD MRD VAF≥0.1% experienced higher rates of relapse (73.8% vs. 20.5%, p < 0.0001) and decreased OS (11.4% vs. 66.8%, p < 0.001) compared to VAF<0.1%.
The highly sensitive SA-NGS method allowed for exploration to determine if a deeper VAF threshold of 0.01% (previously validated for NPM1 and FLT3-ITD in the Pre-MEASURE study) was prognostic for FLT3-TKD as an MRD target. This led to an additional 20 patients being reclassified as positive (n = 34 with VAF above 0.01%, =9.9%). However, there was no significant difference in CIR (33.6% vs. 19.7%, p = 0.089) or OS (65.2% vs. 66.9%, p = 0.267) between patients with 0.01%≤VAF<0.1% and VAF<0.01% ( Figure). Out of 28 SA-NGS positive calls, 6 could not be validated by orthogonal digital droplet PCR testing, and interestingly, none of those 6 patients experienced relapse.
Site-reported pre-transplant remission multiparametric flow cytometry (MFC) was available for 335 patients (98%). Results of MFC were not associated with differences in CIR (HR 1.2, 95% CI 0.52 - 2.76, p = 0.67) or OS (HR 0.78, 95% CI 0.34 - 1.77, p = 0.551).In multivariable analysis, FLT3-TKD NGS-MRD VAF≥0.1% remained prognostic for CIR (HR 6.1, 95% CI 3.1 - 12.0, p < 0.001) and OS (HR 3.2, 95% CI 1.7 - 5.9, p < 0.001).
Conclusions: Detectable persistence of FLT3-TKD variants in blood from AML patients in first remission prior to first alloHCT is rare, but at a VAF level of 0.1% was strongly associated with increased relapse and death after transplant compared to those testing negative. In contrast to FLT3-ITD NGS-MRD, no evidence was found to support lowering the NGS-MRD VAF threshold from 0.1% to 0.01% for FLT3-TKD. Alternative methodology may further improve NGS-MRD test performance. Site-reported MFC prior to alloHCT did not stratify for post-transplant relapse or survival. Optimal NGS-MRD testing for patients with AML will likely require a multi-target approach and testing for persistence of FLT3-TKD MRD is shown here to represent an important component of this strategy.
Disclosures
Andrew:Astra Zeneca: Current Employment. Auletta:National Marrow Donor Program: Current Employment; Takeda: Membership on an entity's Board of Directors or advisory committees; AscellaHealth: Membership on an entity's Board of Directors or advisory committees. El Chaer:Celgene: Research Funding; Amgen: Consultancy, Research Funding; Fibrogen: Research Funding; Sumitomo Pharma Oncology: Consultancy, Research Funding; Sanofi: Research Funding; PharmaEssentia: Research Funding; BioSight: Research Funding; MEI Pharma: Research Funding; Novartis: Research Funding; Arog Pharmaceuticals: Research Funding; Association of Community Cancer Centers: Consultancy; DAVA Oncology: Other: Travel grant; Bristol Myers Squib: Research Funding. Corner:Bio-Rad Laboratories: Current Employment, Current holder of stock options in a privately-held company. Jimenez Jimenez:Abbvie: Research Funding. de Lima:AbbVie: Other: Data Safety Monitoring Board; Novartis: Other: Data Safety Monitoring Board; Miltenyi Biotec: Research Funding; Bristol Myers Scribb: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees. Kebriaei:Pfizer: Consultancy, Honoraria; Jazz: Consultancy, Honoraria. Hourigan:Foundation of the NIH AML MRD Biomarkers Consortium: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal